Experimental Study on the Fuel Requirements for the Thermal Degradation of Bodies by Means of Open-Pyre Cremation
Abstract
The results of a systematic study of open-pyre cremation of bodies is reported here with the aim of providing quantitative information on the mechanisms controlling the cremation process, and the relationship between the characteristics of a fire and the level of consumption of a body. Systematically constructed timber pyres and recently euthanized pig carcasses (as surrogates for human bodies) were used to establish the importance of fuel quantity, methodology of fuel application, body size and body arrangement. The results indicate that a fuel/body mass ratio greater than 9 is necessary to overcome the endothermic effect of the body on the pyre. Even with a fuel/body mass ratio of 9 and ideal burning conditions, full destruction of all organic matter could not be attained.
1. Introduction
For centuries, cremation has been used as a means to dispose of bodies. The reasons encouraging the use of cremation can range from sanitation to religious beliefs or disposal of bodies after criminal acts. Bodies affected by intense heat can also be a source of information. In the event of unwanted fires that result in fatalities, thermally degraded bodies can be a vital piece of evidence. A key element of forensic investigations is the identification of the bodies from the cremation remnants.[1] The capacity to establish the identity of the bodies relates closely to the level of destruction. Dentures and DNA are some of the most important means of identification, and generally allow for the establishment of the identity of the bodies even under very severe burning conditions.[2] The degradation of the bodies has to be correlated with the dynamics of the fire to complete a forensic investigation. Consistency between the level of destruction of a body and the burning efficiency, intensity and duration of possible fires has to be demonstrated to infer the validity of a cremation hypothesis.[1,2]
A very high-profile example where this correlation became key to establishing the validity of a forensic hypothesis relates to what has been referred to as the “Historical Truth” in the case of the 43 disappeared students in Ayotzinapa, Mexico.[3] A forensic investigation concluded that multiple bodies (up to 43 bodies) were cremated in the municipal dump of Cocula. The human remains discovered in the dump showed no remnants of DNA due to the high level of heat exposure. A subsequent expert panel concluded that there was a need to conduct realistic experiments to establish the detailed characteristics of the fire necessary to achieve the observed levels of cremation (i.e. intensity of the fire, amount of combustible materials necessary, etc.).[4] To reach this conclusion, the panel verified that the necessary experimental data was not available. It is not uncommon that, after a fire, those conducting the investigation find that necessary quantitative data is not available. As established by the expert panel,[4] an area where there is little quantitative data is thermal interaction between a fire and those individuals exposed to the fire.
The interactions of a body with a flame are extremely complex, thus empirical data is one of the few means to characterize the manner in which a body can be affected by flames. While information on different forms of cremation seems to populate the literature, none of this information provides quantitative data on the amount of fuel necessary for the complete destruction of a body using an open-air pyre, the necessary burning duration, the effect of multiple-body interactions and the net energy output of a body under different levels of exposure. The present study was conducted to provide adequate data that allows one to infer the interaction between a body and the fuel during the process of open-pyre cremation.
A human body contains on average of 65%–70% water, 20% organic matter and the rest are bones (10%–15%).[5] Incineration aims at the destruction of organic matter, and has to overcome the energy required to vaporize the water. Schmidt and Symes[2] summarize the typical characteristics of human-body cremation. They indicate that, for incineration to be legal, bone residues cannot have organic matter for body identification. The bone remains are fragile, but they generally maintain similarity with their original characteristics. Colour changes to white, and when they are moved away from the furnace, they tend to fracture. Generally, the data shows that the legal incineration of an adult body, in a crematory furnace, requires a temperature between 800°C and 1000°C for 90–120 min. These values vary, according to the literature, because of the variability of bodies,[5] furnaces and fuel to be used.[2] Nevertheless, the reported temperatures and times are mostly within the ranges indicated above.
The design of a crematory furnace is not simple.[6] The design objectives are to maintain a homogeneous temperature, to quickly evacuate degradation products (keep the furnace ventilated) and concentrate the combustion energy to achieve a high efficiency. It is important to emphasize that efficiency is defined on the basis of the amount of fuel necessary to maintain the desired temperature during the cremation period. Water vapour and combustion products have an important effect on the efficiency of the burners of an incineration furnace. The effective elimination of these products from the combustion furnace can ensure a more complete combustion and thus a higher efficiency. Therefore, ventilation is essential to maintain an efficient and homogeneous combustion. Furnaces are lined with low-density refractory bricks, so that the bricks’ surface can rapidly heat to the temperature of the gases, thereby converting all the energy into radiation. This refractory material enables the furnace to provide the body with a homogeneous heat flow ensuring a complete cremation of the whole body.[6]
Combustion in a crematory furnace is generated by gas burners that approximate complete combustion and therefore is highly efficient. Open-air burning, where all the fuel is placed horizontally, is at the other extreme of efficiency. The supply of fuel and air is complex and inefficient.[7] Crematory pyres have been studied using reconstructions that follow traditional practices. Studies show that the temperatures at the core of a pyre may exceed 800°C for several hours. Nevertheless, large amounts of fuel are necessary to maintain the cremation process until most of the organic matter is destroyed.[8] According to McKinley,[8] an ideal crematory pyre design improves combustion efficiency, so that only 700–900 kg of wood are necessary to deliver bones free of organic residues. If the pyre is smaller than the body, then cold air will not allow cremation of the body’s limbs. Therefore, the customary dimensions of a pyre surface are around 2.5 m × 1.5 m.[8] The typical duration for fuel-load consumption is about 6–7h,[8] allowing for 6 or 7 additional hours, during which the corpse is left to be consumed by the embers.[3]
The most well-known tests involving cremation of bodies for forensic investigation purposes were conducted by DeHaan with pig remains,[9] which are summarized in Ref. [1]. Those tests show that, when the body is wrapped in clothes, allowing the carbonization of skin and clothes, they act as a wick enabling subcutaneous fat (approximately 20% of the body mass [5]) to maintain combustion. While some quantitative information is provided, most of the results are qualitative and do not allow one to infer the exact amount of fuel necessary for cremation. An important conclusion is that the net energetic balance of a body is positive with exothermic average heats of combustion of the order of 17 MJ/kg[10] and for body fats of 39.8 MJ/kg.[9] This has commonly lead to the conclusion that, once the fats are released, the combustion of a body can be self-sustained.[3]
An aspect that is not covered in any of these tests is the interaction between the body and the fuel. As explained above, the organic materials in the body have enough energy to evaporate the water, resulting in a positive net heat release rate. Experimental studies have reported that, under specific burning conditions, peak heat release rates of up to 250 kW per body [10] can be attained. Nevertheless, depending on the efficiency of the burning process, the body might deliver a positive or negative net heat contribution to the fuel driving the cremation. The most comprehensive study on this matter is presented by Bohnert et al.,[11] but it only relates to cremation chambers, and does not include the interaction of multiple bodies and the impact of these interactions on the cremation process.
The only quantitative data on multiple-body cremation in open air can be found in the US Department of Agriculture guidelines, where detailed arrangements for animal disposal are described for different animals. In the case of pigs, the report indicates that an approximate amount of 170–200 kg of fuel per carcass is necessary.[12] The fuel was a combination of hay, carbon and timber arranged in a manner that optimizes heat feedback to the carcass. The influence of different-size animals and of multiple-body interactions are described qualitatively. These guidelines do not indicate the level of destruction attained.
Combustion of solid or liquid combustible materials (fats) adhered to non-combustible materials (bones) can be maintained as long as there is enough energy to sustain the gasification of the combustible material.[13] The energy comes from the flame, part of the energy is lost to the environment, and part is transferred to non-combustible materials to which the fuel is adhered. As the combustible material is consumed, the residual combustible material is increasingly lower, thereby reducing the energy generated by the flame, and increasing the fraction of the energy lost to the non-combustible material (bones and water). Finally, the flame is extinguished. Therefore, attainment of complete destruction of organic matter is not only related to the exothermicity of the body, but it is mostly an extinction problem linked to the net heat feedback to the fuel. The literature describes this process of extinction as quenching, and relates it to the need to attain a minimum heat feedback that maintains the flame at a temperature sufficient for combustion to occur (critical mass-transfer number).[13] Heat transfer is configuration dependent, thus very much affected by the burning conditions and geometry. To characterize the extinction process for an open-pyre burning of multiple bodies, it is therefore necessary to study the cremation process in the specific configuration.
Most of DeHaan’s tests [9] were voluntarily extinguished, except for one of the tests in which, after a burning period of almost 4 h, the fire was allowed to extinguish naturally. DeHaan reports that, when the fire fully extinguished (after 6 h), about 50% of body mass (including significant organic residues) remained. Data that enables one to understand the extinction process is therefore not available.
The present study attempts to fill a gap in the literature by reporting on a series of systematic experiments that address complete destruction of organic matter during open-pyre incineration by focusing on extinction. The results include the amount of fuel necessary for the complete destruction of a body using an open-air pyre, the necessary burning duration, the effect of multiple-body interactions, and the net energy output of a body under different levels of exposure.
2. Experimental Setup
Six experiments were conducted in an open field. The experiments consisted of burning pig carcasses on top of a wood pyre (fuel), using different numbers of carcasses and different fuel-to-animal mass ratios (F/A). The pigs were always placed as close as possible to the centre of the pyre, making sure that there was good contact between the carcass and the wood. Details of these experiments are summarized in Table 1. Pig carcasses have been commonly used as surrogates for human bodies, and while differences between organic matter from a pig and a human body are significant,[14] the similarities have been long recognized.[15]
For the experiments with one pig carcass (1–4), a blank test was conducted at the same time, using an identical second pyre, but without a pig carcass on top. The two fires were set at 15 metres apart to avoid feedback or interaction between the two fires. Both fires were ignited at the same time to ensure consistent behaviour. A schematic representation of the experiments’ configuration can be seen in Fig. 1.
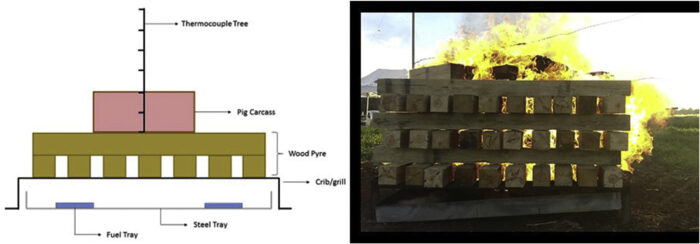
The wood pyre was placed over a metallic support that consisted of three parts: (i) a metal frame constructed from 50 mm L-section steel, (ii) a metal grate that sits inside the frame to prevent wood falling below whilst also providing adequate air entrainment, (iii) a metallic tray below the frame to collect ash and any dripping fats.
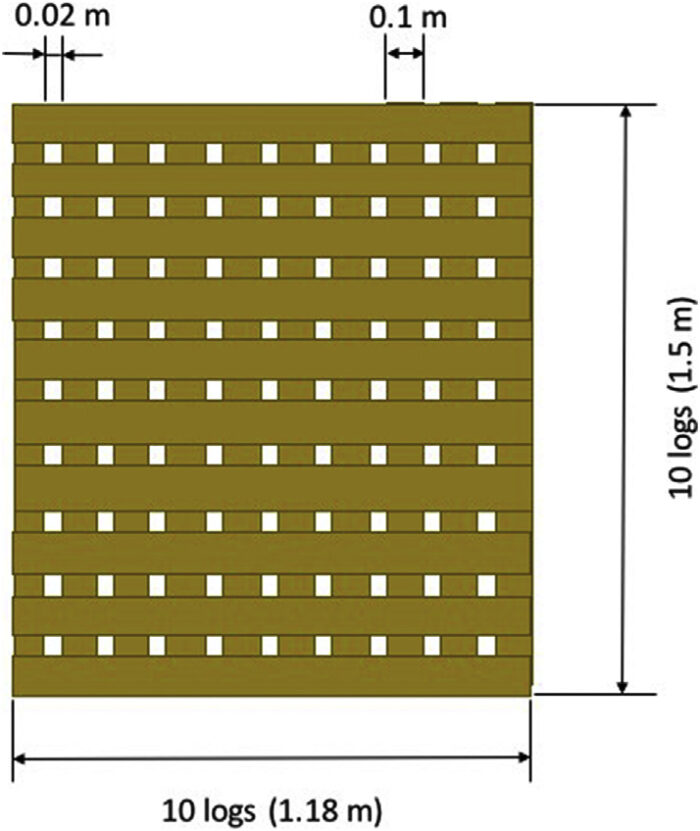
The wood pyres were built using 10 cm square section logs that were 1.5 m long. The logs were placed in cross hatched manner (see Fig. 2), 10 per layer, with a 2 cm gap between each log to allow adequate airflow. Gross[16] identified two regimes of burning corresponding to ‘under-ventilated’ and ‘well-ventilated’ cribs. In the loosely packed regime, the burning rate is more closely approximated by the free burning rate of the individual sticks, and is governed by heat and mass transfer processes near the surfaces. In this regime, the burning rate is more of a function of the stick dimensions, and is independent of the “porosity” of the crib.[17] The log configuration in the cribs used here allow the burn to have high porosity and substantial flaming. In this case, the rate of burning is controlled by the thickness and separation of the individual sticks and the number of stick layers. The length-to-thickness ratio of the wood logs was kept constant throughout the tests with a value equal to 15.[17] The length-to-thickness ratio was chosen to ensure self-sustaining fires and maximum burning rates, thus best possible burning conditions. In this way, pyres were rectangular-shaped of 1.50 m × 1.18 m (see Fig. 2). The height of the pyres varied according to the amount of wood used.
The wood (pine) was characterized by proximate (ASTM E870 – 82) and elemental analysis.[18] C, H and N results obtained by combustion using a LECO TruSpec analyser. Other elemental results for available elements obtained on a Varian Vista Pro ICPOES instrument on samples extracted with Mehlich-3 reagent. The moisture content of the wood was between 14%–19%. Once logs have been cut, the moisture content of wood is a function of thermodynamic equilibrium with ambient conditions, therefore, the wood used for this study can be considered representative of very dry atmospheric conditions, thus ideal for burning. The higher heating value (HHV) was calculated from the elemental analysis results by means of the equation proposed by Friedl et al.[19] This HHV is consistent with literature values measured for pine with similar moisture content (between 16 kJ/g – 20 kJ/g)[20] and approximately 10% less than the heat of combustion of dry pine.[20] A summary of the wood characteristics can be seen in Table 2.
The fires were initiated with a mixture of kerosene/n-heptane placed in 4 containers (commercial baking trays 200 mm × 300 mm) placed inside the metallic tray (see Fig. 1). To sustain the initial fire in the containers for at least 5 min, each tray contained 600 mL of kerosene and 100 mL of n-heptane. The point at which all containers were ignited was considered as the start of the fire.
The animals used were Large White pigs between 53 and 81 kg. The pigs were euthanized humanely approximately five hours before the experiments. Each pig carcass was wrapped in a woollen blanket of 1.8 kg and placed in the centre of the pyre. The blankets simulate clothing and also act as a wick absorbing fat that is being released from the burning carcass.[9,10] When two carcasses were used, these carcasses were placed with 10 cm separation, and ensuring stability of the pyre throughout the fire. When four carcasses were used, the same configuration was used, but placing the carcasses in two layers of two pigs each.
3. Instrumentation
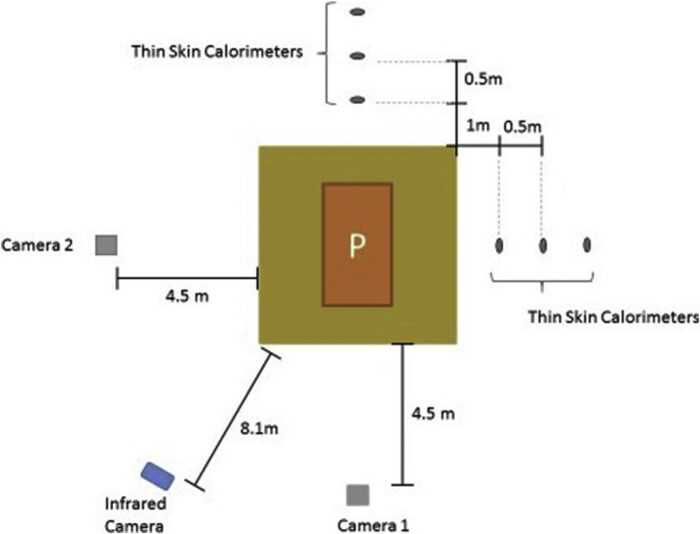
The height of the flames was determined from video recordings and then used to infer the heat release rate. While this is a very approximate method, it can be used for relative comparison of similar tests. The flame-height expression described by Cox and Chitty[21] was used in this study. The height of the flames as a function of time was determined using video cameras. For each pyre, two video cameras (Sony CX405) were placed aligned with the centre of the pyre and perpendicular to each other, as shown in Fig. 3. The camera resolution was set to 720p, and the cameras were placed horizontally at 4.5 m from the edge of the pyre, 1 m above ground. In the case of the pyre with the carcass, an infrared camera (Trotec IC120LV) was setup horizontally 8.1 m from the edge of the wood, also 1 m above ground. The emissivity was set to 0.9.
To determine the height of the flames as a function of time, the video recorded during the fire was processed through Adobe After Effects© and Adobe Premiere Pro© to contrast the flames. A Matlab script was created to determine the height of the flame from every frame of the videos. A series of thresholds was established for the flame/no flame boundary to establish the sensitivity of the correlation to the threshold. It was noted that the sensitivity was weak, and if the Matlab script was run at 1 frame/second and a moving average of 30 measurements was applied, most of the noise was removed from the data.
A thermocouple tree consisting of eight sheathed thermocouples (K-type) was constructed in the centre of the pyre (see Fig. 1). The bottom thermocouple was placed below the top plank, and each following thermocouple was placed in 250 mm increments. One thermocouple was inserted inside the pig’s stomach to measure the internal temperature of the pig throughout the fire.
Eight thin-skin calorimeters (TSC) were placed around each pyre, four on each side as shown in Fig. 3. In each row, the first TSC was placed at 1 m from the pyre, while the other TSCs were placed at 50 cm from the previous. Every TC and TSC was connected to an Agilent 34980A Datalogger and PC for data recording.
4. Estimation of the Heat Release Rate from the Flame Height
The heat release rate as a function of time can be estimated from the wood pyres using an empirical correlation of flame heights. Estimated heat release rate was calculated against various flame-height correlations by Zukoski,[22] Heskestad[23] and Cox and Chitty.[21] Comparison of the estimated and measured heat release rate using oxygen-consumption calorimetry showed that for the cribs the Cox and Chitty [21] correlation was the one that best fitted the data. The correlation establishes that the ratio Lf/D is proportional to Q*², where Lf is the flame height, D is the diameter of the fire (1.5 m) and Q* is the non-dimensional heat release rate. Three laboratory experiments were conducted with different crib sizes to determine the coefficient of proportionality for the specific cribs of this study. The initial size of the crib was approximately 1 m by 1 m, and two and three layers of timber were used. Images and heat release rates were collected throughout the duration of the experiment. The data were processed, and a linear regression of the data was conducted to obtain the gradient achieving the following flame-height correlation:
| Lf | = 9.12 Q*² | (1) |
| D |
For these experiments, the cameras were placed horizontally at a distance of 4 m and 1 m above ground. The mass loss throughout the fire was measured with a scale (Levantina de Pesaje, class 5 cell, OIML approval) and the HRR was calculated by means of Oxygen Consumption (OC) and carbon oxide/carbon dioxide generation (CDG) measurements.[24],[25],[26]
The time interval where the height of the flame was used for this correlation varied within the experiments, but in all cases was approximately between 1000 and 4000 s. Beyond these limits, the size of the fire is small, and the model is not valid. Results showed that this model can estimate the heat released rate with a maximum error of 15% in a range between 100 kW and 1 MW. The configuration of these laboratory experiments was the same as the field experiments, but the crib was placed under a large hood with capacity up to 1 MW. Some of the field experiments led to slightly bigger fires (estimated 1.2 MW) but, since the laboratory hood used could not exceed 1 MW, it will be assumed that the correlation does not yield a larger error in the extrapolation range.
The calibrated correlation presented in Equation (1) was used to calculate the HRR for the tests conducted in the open. In the absence of OC calorimetry measurements, the video recordings and Equation (1) served as a means to establish the HRR.
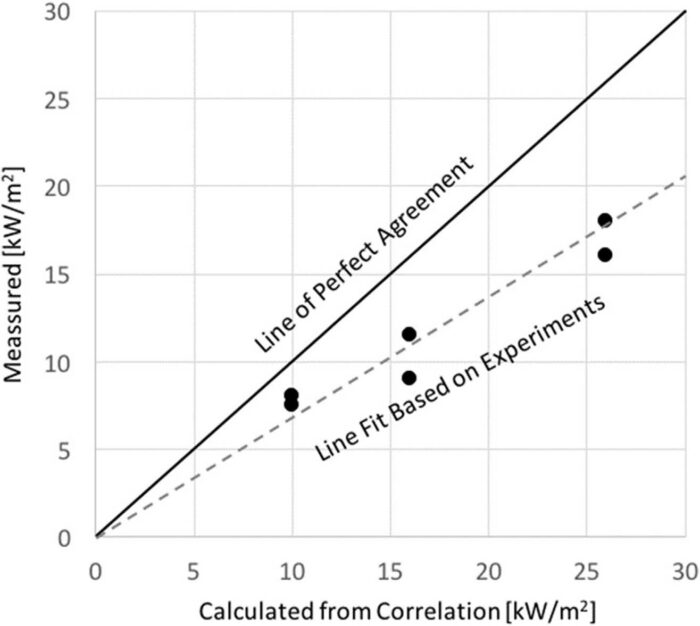
A final verification was conducted with heat-flux measurements by comparing the results to those reported by Koseki[27] for large pool fires. As described before, heat-flux measurements were conducted at different distances from the pyres for both the pyre with the pigs and the blank. As can be seen in Fig. 4, the heat-flux measurements follow a similar trend but generally slightly lower than those reported by Koseki.[27] A deviation of 46% was found on the slope, but the differences were not significant enough to infer a complete deviation from pool-fire behaviour.
5. Experimental Observations and Results
Each experiment and the equivalent without the carcass were ignited simultaneously. Video recordings, temperature measurements and the heat-flux measurements were acquired throughout the duration of the tests. All tests were allowed to burn until extinction occurred in a natural manner.
Fig. 5 shows the temperature histories for a single animal and a ratio F/A = 2 (Experiment 1). The surface of the timber corresponds to zero, with negative below and positive above the timber surface. The animal acts as a heat sink that is sufficient to prevent the spread of the flame, temperatures are very low, and only after about 4000 s the flames manage to creep around the carcass (Thermocouple at -10). The flame extinguishes at approximately 100 min, leaving significant amounts of timber unburnt. The timber continued to smoulder around the carcass but extinguish underneath the animal. A significant amount of organic matter was left after extinction. The blank showed slightly higher temperatures and continued to burn until all the timber was mostly consumed. For a F/A = 2, the animal carcass acts as a heat sink that the burning timber cannot overcome when the wood-char thickness increases and burning rates decrease. This experiment shows that self-sustained open burning that lasts sufficiently long to consume the entirety of the organic matter with this F/A ratio does not seem possible. The heat supplied by the pyre is not sufficient to enable the carcass to attain self-sustained combustion.
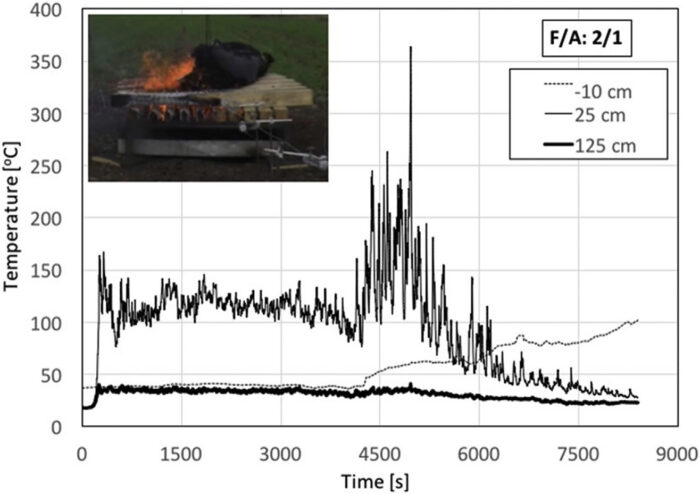
Fig. 6 shows the temperature histories for a single animal and a ratio F/A = 5. The solid line corresponds to the test with the animal, and the dotted line to the blank test. While significant temperature fluctuations were recorded, it can be seen that temperature inside the crib (-10 cm) is consistently lower in the presence of the carcass showing that, throughout the test, the carcass has a negative thermal contribution to the crib. For the crib with the animal carcass, temperatures above and below the surface of the crib are comparable showing the important effect of the thermal mass of the animal on the behaviour of the ensemble. The fire never managed to fully engulf the crib, burning mostly as small flames surrounding the carcass. The combustion of the organic matter produces sufficient energy to maintain gas-phase temperatures fairly homogeneous up to 125 cm from the crib surface. Temperatures measured by the thermocouples remained in the 200–250°C range, which is significantly below what has been normally observed for a crib.[16] For the blank crib, the flames first establish within the crib burning with a small flame height. The temperature 125 cm above the crib surface remains low for more than 3000 s, but eventually, when the crib is fully engulfed in flames, temperatures reach values consistent with those typically reported for wood cribs.[16] It is important to note that, by the time the blank crib reaches its maximum burning intensity, the crib with the animal carcass has already progressed towards extinction. The carcass was observed to still retain significant amounts of visible organic matter after extinction. For F/A = 5, it was also observed that self-sustained open burning that lasts sufficiently long to consume the entirety of the organic matter does not seem possible. While sustained combustion exists while the timber is burning, the carcass is unable to sustain burning once flaming combustion of the timber has ceased.
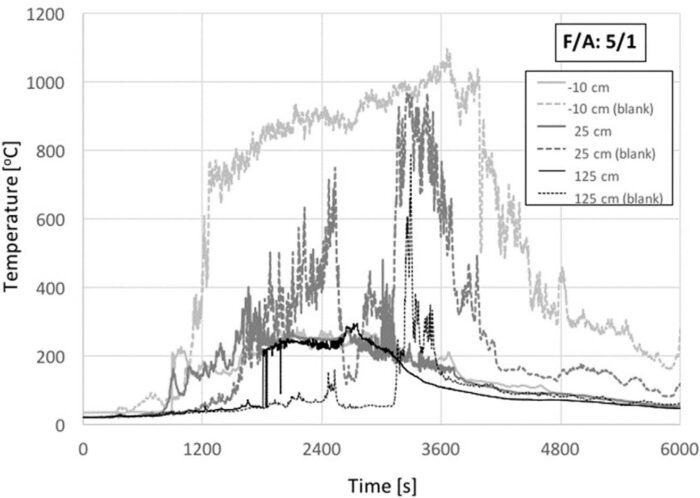
Fig. 7 shows the temperature histories for two animals and an F/ A = 5. The temperatures at 25 cm above the crib surface increase rapidly as the animals get involved in the fire. The temperatures nevertheless do not reach the same peak values as for one animal carcass. The thermocouple 125 cm above the crib surface remains cold through the duration of the burning, because the fire never progresses from small flames surrounding the carcasses. Instead, in the interior of the crib the temperatures are higher. This is mostly because the crib is deeper, therefore it is burning strongly below the thermocouple. Burning decays to smouldering much faster than for the single carcass (~2500 s as opposed to ~4000 s). Temperatures within the interior of the crib (-10 cm) continue to increase as a vigorous smouldering reaction establishes underneath the carcasses. Eventually (~5200 s), the crib collapses resulting in an increase in temperature for a short period of time. Once the crib has collapsed, the fire progresses slowly towards extinction, as the temperature histories indicate. Increasing the amount of available fuel by increasing the volume of the crib results in preferential burning in areas not exposed to the heat sink of the carcasses. Enhanced fuel consumption results in loss of mechanical integrity and collapse under the weight of the carcasses. Once the crib has collapsed and the void fraction decreases, extinction follows. As in the previous experiments, a significant amount of organic matter was left after extinction, and the carcasses are unable to sustain burning, once flaming combustion of the timber has ceased. The presence of two animals results in different burning characteristics but does not strengthen the combustion process.
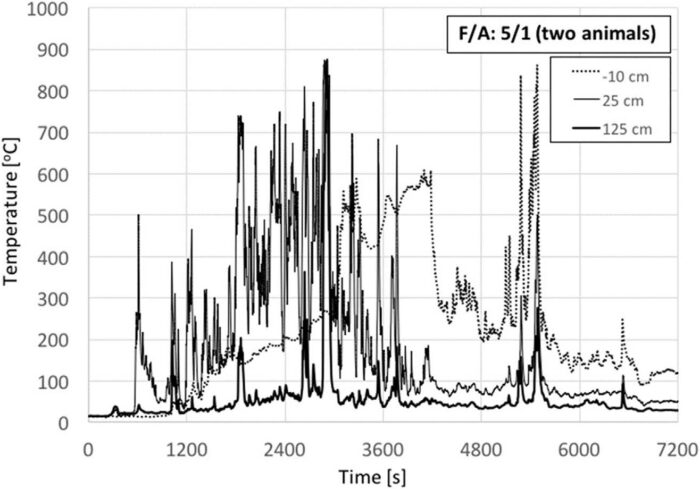
Fig. 8 shows the temperature histories for four animals and also a F/A = 5. In this case, fuel was not placed fully underneath the carcass but only a fraction of the total fuel was placed initially and then the rest was added continuously every time the flame heights started to decay. This process was followed to increase the efficiency of the heat feedback from the timber to the animal carcass while still using practises that could be considered common in open-air burning. Furthermore, by not increasing the volume of the crib, preferential burning and mechanical collapse was avoided, and the fire allowed to burn for much longer.
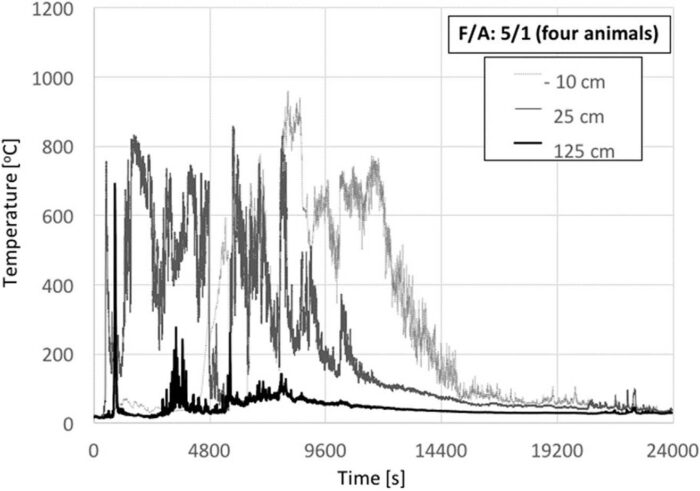
As it can be seen from Fig. 8, and consistent with the results of Fig. 7, in the absence of additional fuel, the temperatures show a rapid decay until new fuel is added (~1500 s, ~3000 s, ~4500 s, 6000 s, etc.). As the fresh fuel starts to burn, temperatures increase again. This repeats itself during the test every time new fuel is added. This shows that a continuous delivery of fuel can maintain burning (fresh fuel has no char, thus burns more vigorously [7]) and burning timber above the animal carcass improves the net heat feedback from the flame to the animal. Temperatures up to 25 cm above the crib consistently reach higher values than for previous tests. It is important to note that, with four carcasses, the decay is much faster than with two. Within less than 800 s, temperatures start to decay, only increasing once the new fuel is added. This indicates that an increase in the number of animals does not have a positive effect on the crib, even under improved burning conditions. Burning for this test is characterized by small flames and therefore low temperatures. Fig. 7 shows that the temperature decays almost to ambient at 125 cm above the crib surface. Once the available fuel was exhausted, the fire further decreased in size (as shown by the temperatures 25 cm above the crib surface) and slowly decayed towards extinction. After extinction, it was observed that significant organic matter remained on the carcasses.
The animals chosen for this test were smaller, which has been previously reported as having a positive impact on the overall energy balance.[12] Smaller animals were chosen to be able to maintain them within the burning surface of the crib, but also to evaluate the role of multiple animals under improved burning conditions. It can therefore be concluded that increasing the number of animals does not have a positive effect on the crib at this F/A = 5 ratio, even if the animals are smaller. This is an important conclusion because it suggests that, for this F/A ratio, to establish the minimum amount of fuel required for incineration of multiple animal carcasses, it is not necessary to conduct experiments with more animals. The minimum amount of fuel required will be that necessary for a single animal because the carcass to carcass interactions are detrimental to the fire.
Once the ratio has been increased to F/A = 9 (Fig. 9) at the onset of burning, the two pyres burn almost identically. The pyre with the animals showed initially (first 2000 s) higher flame heights as reflected by higher temperatures at 125 cm. The flame rapidly establishes around the animals decreasing in height and progressing towards an ensemble of small flames, as evidenced by the temperature decay measured at 125 cm. Eventually, the blank reaches higher temperatures for all three thermocouples. The blank will continue to burn longer than the pyre with the animal which cools rapidly once flaming combustion has ceased. This shows that the carcass is still acting as a mild heat sink. The burning was allowed to proceed until extinction occurred, and even for F/A = 9 there was significant visible organic residue. For a F/A = 9, the fire approaches conditions under which an open pyre can attain self-sustained burning of the ensemble until the total consumption of the organic matter on the carcass. While self-sustained burning was not fully achieved, it was not possible to conduct tests with a higher value F/A ratio. Thus, for the purpose of these tests, it will be established that, for self-sustained open burning of a single animal carcasses, it is necessary to have at least an F/A > 9. Multiple carcasses will require a larger F/A ratio.
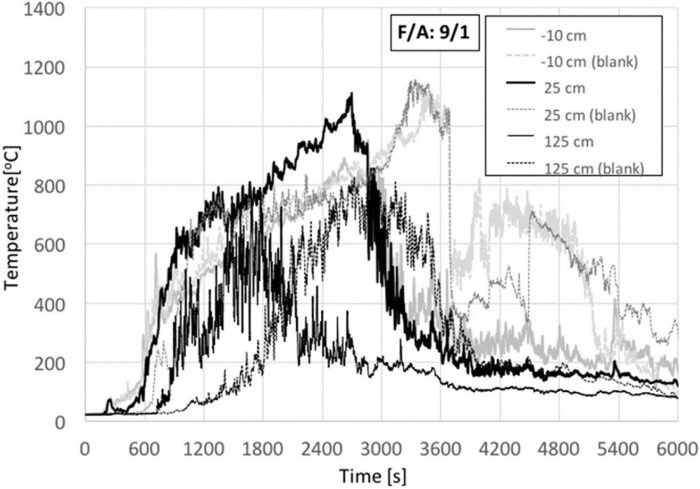
Fig. 10 shows the calculated heat release rate for an experiment with F/A = 3. Both experiments were ignited at the same time (t = 0) but data is only presented once the kerosene/n-heptane was fully consumed and only the crib is burning. Before consumption of the kerosene/n-heptane, the data is presented as zero. The shield provided by the carcass and the ignition of the blanket results in earlier consumption of the kerosene/n-heptane and ignition of the crib. Initially, the crib burns vigorously, followed by a slow decay period. Eventually, flaming will disappear, and only smouldering of the wood remains. At this stage, the methodology to establish the heat release rate is no longer valid, so the heat release rate data is eliminated at the onset of smouldering. The onset of smouldering is indicated in Fig. 10, and the data after that is presented as zero. The heat release rate is smaller for the crib with the carcass through the integrity of the experiment, nevertheless, the transition to smouldering occurs first for the blank. More vigorous burning results in earlier consumption of the wood and a faster transition to flaming. In contrast, smouldering lasts much longer in the absence of a carcass. The carcass acts as a heat sink, eventually extinguishing the smouldering embers.
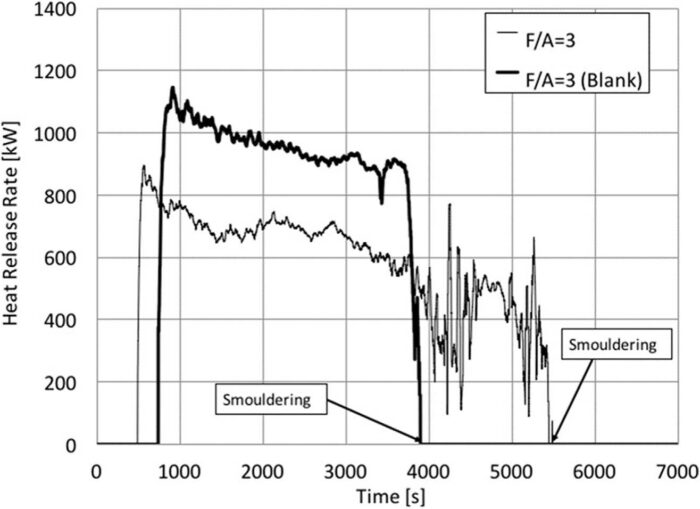
Table 3 presents a summary of the duration times of each experiment. In the cases with the lower F/A ratios (i.e. 2 and 3) the flames self-extinguish before total consumption of the wood. The blanks burnt for a longer period of time, until total consumption of the wood was observed. In all other cases, there were mostly ashes left when the experiment concluded. Table 3 shows that the shallow cribs will have a much lower burning rate than the deeper cribs (as previously reported in the literature [16]) and therefore, the impact of the animal carcass on the burning of the crib diminishes as the height of the crib increases. For F/A = 5 and F/A = 9, the duration of burning is only slightly longer for the crib with the animal carcass, indicating a minor negative impact of the carcass on the crib. These numbers are only presented as a reference, because the animals had all different weights, therefore the quantity of wood changed, even if the F/A ratio was to remain equal. The overall duration of burning was found to be consistent with other open-air cremation data.[11]
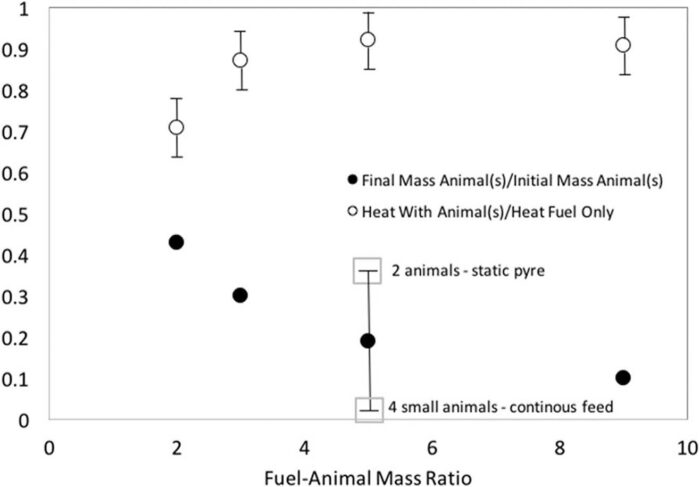
Fig. 11 shows the ratios of residual mass to initial organic mass, and the ratio of heat release rate between the pyre with the animal and the blank. The amount of bones is subtracted from the mass of remnants, normally approximately 13% of the mass corresponded to the bones, 8% in small fragments and 5% in almost intact bones. While some variation was observed from test to test, the values subtracted were always the same. The error bar showed in the mass ratio of the experiment with fuel-animal mass ratio 5 corresponds to the interval obtained for the 3 experiments carried out at the same F/A (using 1, 2 and 4 pig carcasses). The single animal experiment is the baseline case, while the two-animal experiment shows how adding a second animal results in more residual organic mass, while optimizing burning by reducing the size of the animals and actively supplying the fuel can almost eliminate all organic matter. These results show that the F/A alone cannot define the residual organic mass; nevertheless, even under optimal burning conditions an F/A > 5 is required for total consumption of the organic mass. The total heat released was calculated for the experiments and blanks as the average of the heat release rate determined from the height of the flames as a function of time.
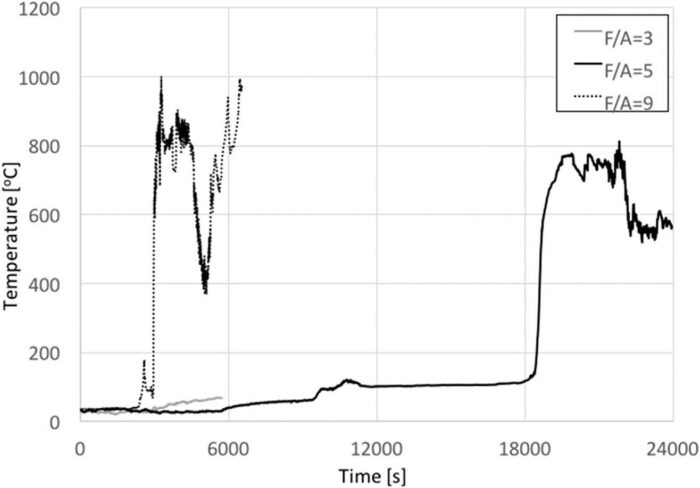
Results showed that the mass of carcass left after the experiment decreases with F/A. In all cases, there was a considerable amount of flesh in the carcass remains, as it can be seen in Fig. 12. Even using a F/A = 9 (which corresponds to 630 kg of wood for an average body of 70 kg) there was flesh and organs left. Fig. 13 shows the temperatures recorded by a thermocouple in the interior of the pig. The thermocouple placement is uncertain given the nature of the experiment, thus these temperatures are only quoted for qualitative purposes. It is important to note that the literature cites temperatures of approximately 300°C as a limit for successful DNA recovery.[28] For F/A = 3, temperatures never exceeded 100°C. For the ideal case of small animals, continuous heating and F/A = 5, the thermocouple became exposed after approximately 5 h, showing a drastic increase in temperature. A similar observation could be made for the F/A = 9, where this change occurs at approximately 50 min. Survival of DNA is therefore guaranteed for F/A = 3. Teeth were removed to conduct before/after DNA testing; nevertheless, given the amount of organic matter left in all cases studied, it was clear that residual DNA was left, therefore there was no need for these tests.

Finally, the ratio of heat released experienced an increase with the fuel load, and seems to be constant above a F/A of 5. In all cases, the ratio of heat released remains below 1, which means the animal carcass is always acting as an energy sink. This observation is important, because it verifies that, even if exposed to heat for a long time when cremated in open pyres, the efficiency of heat exchange between the flames and the fuel source is such that animal carcasses cannot sustain burning in the absence of sufficient external heating. In the past, it has been reported that animal-skin burning results in a net exothermic heat generation, thus the implication is that self-sustained burning is possible. Measurements using a cone calorimeter have indicated that the effective heat of combustion of animal skin is approximately -27 MJ/kg, with an incident radiant flux of 35 kW/m², and -32 MJ/kg with an incident radiant heat flux of 50 kW/m².[10] The significant difference between the effective heat of combustion shows that, as the external heat flux increases, the composition of the effluent of pyrolysis is changing in a manner favorable to combustion. Given the nature of water gasification and pyrolysis of fats, it can be speculated that, as the net heat feedback decreases, the fraction of water in the effluent will increase, decreasing the effective heat of combustion. Characterization of the effluent composition was unfortunately not possible for this study; nevertheless, given the gas-phase temperatures, the net heat feedback to the exposed face of the animal carcass can be assumed to be significantly below 35 kW/m².[21, 27] Flame quenching will occur as the burning rate decreases below a certain critical value, but the critical value will be defined not only by the total burning rate but also by the water content of the effluent. This process of quenching has been characterized in the past as a critical mass loss [7] or critical mass transfer number [13] for ignition/extinction. Self-sustained burning is therefore not only a function of the heat of combustion, but also of the net heat balance at the skin surface.
6. Conclusion
A series of experiments, using pig carcasses as surrogates for human bodies, were conducted to establish the conditions that will result in total destruction of organic matter in the cremation of bodies by means of an open pyre. The following conclusions have been reached:
- As the net heat supply to the animal surface decreases, combustion supported by the degradation of animal carcasses ceases because of flame quenching associated with the reduced generation of combustible gases.
- A minimum of nine times the weight of the body in dry wood is necessary to achieve almost complete destruction of all organic matter (<10%) when the pyre is left unattended.
- Under ideal conditions (smaller carcasses and continuous feeding of fuel) a minimum of 5 times the weight of the body in dry wood is necessary to achieve almost complete destruction of all organic matter (<10%).
- For all conditions studied, the presence of a carcass will always result in weakening of the fire, but will not affect the structure of the flames significantly. Only if the amount of fuel is very small (F/A = 2) then the heat sink associated to the carcass will reduce the fire size to a point where flame extinction occurs.
- Carcass to carcass interactions with the pyre result in a stronger endothermic impact of the carcass on the crib, thus it is less efficient to cremate multiple carcasses than a single carcass.
- Self-sustained burning of animal carcasses in an open-pyre configuration is not possible. Significant energy from the wood is always necessary to avoid quenching.
All estimates provided in the above conclusions are conservative given that, in all cases studied, significant organic matter was still left in all the animals cremated.
Acknowledgments
Authors would like to acknowledge all the staff at QASP, The University of Queensland, especially Mark Bauer and Milou Dekkers for providing the carcasses, the site and tools. This work would not have been possible without their advice and kind help with safety measurements and paperwork. Support for this work was provided internally by the Fire Group at the University of Queensland. This work was not part of any commissioned forensic investigation.
Article Information
Corresponding author: Luis Yermán; e-mail addresses: l.yermanmartinez@uq.edu.au, luyerman@gmail.com (L. Yermán).
Keywords: cremation of bodies, incineration, self-sustained burning
Republished and reprinted unchanged (except for a few fixed typos) from Fire Safety Journal, Vol. 98 (2018), where it appeared with the same authors and title on pages 63-73, https://www.sciencedirect.com/science/article/abs/pii/S0379073808003800; with permission from Elsevier Ltd. with license no. 5851480021788 of Aug 17, 2024.
Original’s publication history: https://doi.org/10.1016/j.firesaf.2018.04.007; received 14 February 2018; received in revised form 10 April 2018; accepted 15 April 2018 0379-7112; © 2018 Elsevier Ltd.
References
| [1] | J.D. DeHaan, D.J. Icove, Kirk’s Fire Investigation, seventh ed., Pearson Education Inc., 2012, p. 619. |
| [2] | C.W. Schmidt, S.A. Symes (Eds.), The Analysis of Burned Human Remains, Academic Press, 2008. |
| [3] | E. Illades, La noche más triste: La desaparición de los 43 estudiantes de Ayotzinapa, Grijalbo, 2014. |
| [4] | L. Wade, “Burning bodies’ experiment casts doubt on fate of missing Mexican students,” Science 353 (6305) (16 September 2016) 1191; https://www.science.org/doi/10.1126/science.353.6305.1191. |
| [5] | K.J. Ellis, “Body composition of a Young multi ethnic male population,” Am. J. Clin. Nutr. 63 (1997) 1323–1331; https://www.sciencedirect.com/science/article/pii/S0002916523181081. |
| [6] | J.J. Schultz, M.W. Warren, J.S. Krigbaum, “Analysis of Human Cremains: Gross and Chemical Methods,” in: J.I. McKinley, in: C.W. in Schmidt, S.A. Symes (Eds.), The Analysis of Burned Human Remains, Academic Press, 2008, pp. 75–94. |
| [7] | D.D. Drysdale, Introduction to Fire Dynamics, third ed., John Wiley and Sons, 2011, p. 130. |
| [8] | J.I. McKinley, in: C.W. in Schmidt, S.A. Symes (Eds.), The Analysis of Burned Human Remains, Academic Press, 2008, pp. 163–184. |
| [9] | J.D. DeHaan, S.J. Campbell, S. Nurbakhsh, “Combustion of animal fat and its implications for the consumption of human bodies in fires,” Sci. Justice 39 (1999) 27–38; https://www.sciencedirect.com/science/article/abs/pii/S1355030699720113. |
| [10] | A.M. Christensen, “Experiments in the combustibility of the human body,” J. Forensic Sci. 47 (3) (2002) 466– https://asmedigitalcollection.asme.org/forensicsciences/article-abstract/47/3/466/1185251/Experiments-in-the-Combustibility-of-the-Human. |
| [11] | M. Bohnert, T. Rost, S. Pollak, “The degree of destruction of human bodies in relation to the duration of the fire,” Forensic Sci. Int. 95 (1998) 11–21; https://www.sciencedirect.com/science/article/abs/pii/S0379073898000760. |
| [12] | National Animal Health Emergency Management System Guidelines, Operational Guidelines, Disposal, U.S. Department of Agriculture, April 2005. |
| [13] | J.L. Torero, T. Vietoris, G. Legros, P. Joulain, “Estimation of a total mass transfer number from stand-off distance of a spreading flame,” Combust. Sci. Technol. 174 (11–12) (2002) 187–203; https://www.tandfonline.com/doi/abs/10.1080/713712953. |
| [14] | A.M. Barbero, H.F. Frasch, “Pig and Guinea pig skin as surrogates for human in vitro penetration studies: a quantitative review,” Toxicol. Vitro 23 (2009) 1–13; https://www.sciencedirect.com/science/article/abs/pii/S0887233308002658. |
| [15] | A.R. Moritz, F.C. Henriques, “Studies of Thermal Injury: II. The relative importance of time and surface temperature in the causation of cutaneous burns,” Am. J. Pathol. 23 (5) (1947 Sep) 695–720. |
| [16] | D. Gross, “Experiments on the burning of cross piles of wood,” J. Res. Natl. Bur. Stand. 66C (1962) 99–105: https://nvlpubs.nist.gov/nistpubs/jres/66C/jresv66Cn2p99_A1b.pdf. |
| [17] | S. McAllister, M. Finney, “Burning rates for wood cribs with implications for wildland fires,” Fire Technol. 6 (2016) 1–23. |
| [18] | ASTM E870-82, Standard Test Methods for Analysis of Wood Fuels, 2013. Philadelphia. |
| [19] | A. Friedl, E. Padouvas, H. Rotter, K. Varmuza, “Prediction of heating values of biomass fuel from elemental composition,” Anal. Chim. Acta 544 (2005) 191–198; https://www.sciencedirect.com/science/article/abs/pii/S0003267005000735. |
| [20] | M. Aniszewska, A. Gendek, “Comparison of heat of combustion and calorific value of the cones and wood of selected forest tree species,” Leśne Prace Badawcze (Forest Research Papers) 75 (3) (2014) 231–236; https://www.academia.edu/15714042/Forest_Research_Papers_Vol_75_3_2014. |
| [21] | G. Cox, R. Chitty, “Some source-dependent effects of unbounded fires,” Combust. Flame 60 (1985) 219–232; https://www.sciencedirect.com/science/article/abs/pii/0010218085900276. |
| [22] | E.E. Zukoski, “Properties of fire plumes,” in: G. Cox (Ed.), Combustion Fundamentals of Fire, Academic Press, 1995. |
| [23] | G. Heskestad, “Peak gas velocities and flame heights of buoyancy-controlled turbulent diffusion flames,” in: Symposium (International) on Combustion, vol. 18, 1981, pp. 951–960; https://www.sciencedirect.com/science/article/abs/pii/S0082078481800999. |
| [24] | P. Hinkley, H. Wraight, A. Wadley, “Rates of Heat Output and Heat Transfer in the Fire Propagation Test,” Fire Research. Note No. 709, Fire Research Station, Borehamwood, UK, 1968. |
| [25] | C. Huggett, “Estimation of rate of heat release by means of oxygen consumption measurements,” Fire Mater. 4 (1980) 61–65; https://onlinelibrary.wiley.com/doi/10.1002/fam.810040202. |
| [26] | ASTM E1354, Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter, 1995. Philadelphia. |
| [27] | H. Koseki, “Large scale pool fires: results of recent experiments,” in: Fire Safety Science – Proceedings of the Sixth International Symposium, 1999, pp. 115–132. |
| [28] | D.H. Ubelaker, “The forensic evaluation of burned skeletal remains: a synthesis,” Forensic Sci. Int. 183 (2009) 1–5; https://www.sciencedirect.com/science/article/abs/pii/S0379073808003800. |
Bibliographic information about this document: Inconvenient History, 2024, Vol. 16, No. 3; republished and reprinted unchanged (except for a few fixed typos) from Fire Safety Journal, Vol. 98 (2018), where it appeared with the same authors and title on pages 63-73 (https://nsarchive.gwu.edu/sites/default/files/2022-01/Fire%20Safety%20Journal%20%282018%29.pdf); with permission from Elsevier Ltd. with license no. 5851480021788 of Aug 17, 2024.
Other contributors to this document:
Editor’s comments:
